Aquachem Inc.
Canton, MI

|
Plating wastewater contains heavy metals, oil and grease and suspended solids at levels that might be considered hazardous to the environment and could pose risks to public health. Heavy metals, in particular, are of great concern due to their toxicity. Because of the high toxicity and corrosiveness of plating waste streams, plating facilities are required to pretreat wastewater prior to discharge in accordance with National Pollutant Discharge Elimination System (NPDES) permits as required by the Clean Water Act (CWA).
The plating process typically involves, alkaline cleaning, acid pickling, plating, and rinsing. Copious amounts of wastewater are generated through these steps, especially during rinsing. Additionally, batch dumping spent acid and cleaning solutions contributes to the complexity of waste treatment.
With greater quantities of wastewater produced and discharge standards becoming increasingly more stringent, there is a need for more efficient and cost-effective methods for removing heavy metals. Table I shows current Pretreatment Standards for Existing Sources (PSES) for Electroplating Effluent as well as standards under the proposed MP&M rule (Metal Products and Machinery Effluent Guideline).
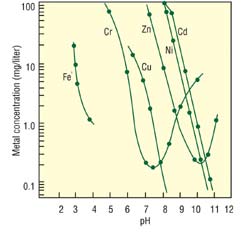
Conventionally, precipitation has been the method most often used to remove heavy metals. Of the few precipitation methods, hydroxide and sulfide are the two main methods currently used, and hydroxide precipitation is by far the most widely used method. However, this method does not ensure total compliance for the various metals present in the waste stream, since all metal hydroxides do not completely precipitate at a single pH. Theoretical hydroxide precipitation curves for metals normally found in plating baths are shown in Figure 1. The graph indicates that there is no one ideal pH for a multiple-metal system.
|
| 1. Precipitation of heavy metals as hydroxides |
Often, post treatment polishing with scavengers such as dithiocarbamate or sulfide, both of which are noxious and toxic, is required in order to meet discharge standards. Table II shows typical concentrations achievable by hydroxide precipitating agents. Such concentrations are obtainable for the individual metal at the corresponding optimum pH. In practice, hydroxide precipitation is generally carried out at the best compromised pH for metals in the waste stream, normally between 8.5 - 9.5. Therefore, achievable concentrations may not meet discharge requirements. Additionally, the presence of chelating or complexing agents, oil and grease, synergistic reactions and high concentrations of dissolved solids can result in diminished efficiency of hydroxide precipitation.
The hydroxide precipitation treatment employs alkaline materials such as caustic soda, soda ash, lime, magnesium hydroxide or a combination thereof. The entire treatment involves precipitation of heavy metal hydroxide(s), flocculation with a polymeric material, settling and discharge of treated effluent. Such a technique is time consuming and requires extensive setup. Each step takes place in a separate tank, and the entire treatment requires several pH adjustments and the use of chemicals such as acids, alum, ferrous and ferric salts, lime or caustic and polymeric flocculent. In addition, the process generates large volumes of sludge/waste that require disposal and is normally hazardous due to the high concentrations of heavy metals. Hazardous waste must undergo treatment to render it suitable for disposal. This treatment adds to the already high cost of wastewater treatment and places an extra burden on treatment facilities.
Case History
A Michigan-based plating facility generates a waste stream that contains high levels of heavy metals, suspended solids and oil and grease. Chelating/complexing agents also enter the waste stream from acid and alkaline cleaning operations. The facility has a treatment system comprised of a neutralization tank, mix tanks, precipitation tank, which includes a precipitation chamber where caustic is fed into the system and a final pH adjustment chamber, flocculent flash mix tank, clarifier, sludge holding tank and filter press. The wastewater is a mix of streams from alkaline cleaning, pickling, rinsing and occasional batch dumps. The treatment runs continuously at 100 gpm, 12-16 hr daily.

|
| 2. Diagram of the existing wastewater treatment setup. |
Previously, the facility used hydroxide precipitation, combining caustic and magnesium hydroxide (Mg(OH)2). Caustic and Mg(OH)2 were added separately to the equalization/neutralization tank where pH was maintained at 7. Due to the slow reaction of magnesium hydroxide, three mix tanks were used. Caustic was added to the first chamber of the precipitation tank to raise the pH to 9.0-9.5 and to precipitate metal hydroxides. After precipitation, the pH was adjusted to about 9.0-9.5. To facilitate sedimentation in the clarifier, a polymeric flocculent was added to the waste stream in the flash mixing chamber. Treated effluent overflowed from the clarifier to a holding tank where it could be sampled and released to a POTW.
Accumulated sludge in the clarifier was withdrawn periodically to the sludge holding tank and finally dewatered in a filter press. Resulting solid waste was hauled away to a disposal site (see Figure 2).
Handling Mg(OH)2 was laborious, and the slurry required continuous agitation to prevent caking in the tank. The system required frequent servicing due to poor performance of the clarifier and inefficient dewatering of generated sludge. The treatment demanded continuous adjustments of the polymer to minimize solid carry-over in the effluent and frequent cleaning of clogged filter cloths.
Alternative Treatment
The conventional treatment was replaced by a proprietary treatment agent, Aquasil®, based on fast kinetics and synergism. Preliminary jar tests indicated that a dose of 900 ppm of the product was sufficient to bring the concentrations of iron, zinc, chromium, oil and grease and suspended solids below discharge. In light of the quality of treated water, the facility recommended a one-month trial. After evaluating the quality of treated effluent, clarifier performance, labor requirements and overall treatment cost, the new treatment was adopted.
The product was applied to the existing setup without reconfiguration, and it eliminated the use of Mg(OH)2. The product is slurried (18% slurry) in the original Mg(OH)2 slurry tank and metered into the neutralization tank. With this approach, use of caustic in the treatment was reduced by 50%. The need for post treatment pH adjustments became infrequent, and use of the polymeric flocculent, although still in use, could be completely eliminated. The product reacts simultaneously with metals, suspended solids and oil and grease to form large, dense floc that settles efficiently, resulting in improved performance of the clarifier, shorter filter press cycles and drier filter press cakes. Treated water is discharged to a municipal POTW, and sludge is dewatered in a filter press.
The current treatment was implemented in June 1998. Since then it has brought about and maintained compliance with discharge requirements. At present, the dose stands at about 200 ppm, yet it continues to provide the same performance with respect to the quality of effluent, clarifier performance and filter press cycles. The high uptake capacity is manifested in the quality of treated water (see Table III). The low content of suspended solids, oil and grease and residual metals contribute to a much lower loading in the sewer.
Waste Disposal
Waste produced by the current treatment contains about 50-60% solids and as such has less volume. The waste is already stabilized and passes the Toxicity Leaching Procedure, TCLP (see Table IV).
Conversion to the current treatment was achieved without system reconfiguration or capital expenditure. The replacement of Mg(OH)2 has eliminated the problems associated with its handling and reduced caustic consumption by 50%. The treatment significantly improved clarifier performance and shortened filter press fill cycles, reducing labor, maintenance and energy costs. Aside from occasionally feeding product to the slurry tank and emptying the filter cake bin, the operator is free to do other tasks.
The treatment proved very economical and versatile, and the product is easy to work with and safe to handle. The new treatment produces effluents that meet or exceed discharge standards and generates nonhazardous waste. Importantly, the facility has realized a 10% annual cost saving for its wastewater treatment.
| TABLE I—EPA Pretreatment Standards for Existing Sources (PSES) for Electroplating Category and MP&M Limits | |||
| Parameter | Daily Maximum (mg/liter) |
Max. Daily Average
for 4 Consecutive Days (mg/liter) |
Proposed MP&M
Daily Maximum (mg/liter) |
| Total Cadmium | 1.2 | 0.7 | 0.21 |
| Total Chromium | 7.0 | 4.0 | 1.30 |
| Total Copper | 4.5 | 2.7 | 1.30 |
| Total Lead | 0.6 | 0.4 | 0.12 |
| Total Nickel | 4.1 | 2.6 | 1.50 |
| Total Silver* | 1.2 | 0.7 | 0.15 |
| Total Zinc | 4.2 | 2.6 | 0.35 |
| Total Cyanide | 1.9 | 1.0 | 0.21 |
| Total Suspended Solids | 20.9 | 13.4 | |
| pH | 7.5 - 10.0 | 7.5 - 10.0 | |
| *Limit applies only to precious metal facilities | |||
| TABLE II: Typical Concentrations Obtainable through Conventional Chemical Precipitation | ||
| Heavy Metal | Achievable Concentration (mg/liter) | Precipitating Agent |
| Cadmium | 0.3 | Soda Ash |
| Trivalent Chromium | 0.5 | Caustic, Lime |
| Copper | 0.5 | Caustic, Lime |
| Iron | 1.0 | Caustic, Lime |
| Nickel | 0.5 | Soda Ash |
| Zinc | 0.5 | Caustic, Lime |
| TABLE III: Analytical Results for Raw and Treated Water | |||
| Parameter | PSES Daily Maximum (mg/liter) |
Before (mg/liter) |
After (mg/liter) |
| Total Cadmium | 1.2 | ND | ND |
| Total Chromium | 7.0 | 4.37 | 0.11 |
| Total Copper | 4.5 | 1.08 | 0.44 |
| Total Iron | N/A | 73.2 | 0.83 |
| Total Lead | 0.6 | ND | ND |
| Total Nickel | 4.1 | 3.35 | 0.07 |
| Total Silver | 1.2 | ND | ND |
| Total Zinc | 4.2 | 221 | 1.86 |
| Total Cyanide | 1.9 | ND | ND |
| TSS | 20.0 | 710 | 13 |
| BOD | N/A | 99 | 27 |
| Total Phosphorus | N/A | 2.0 | ND |
| Total Oil & Grease | N/A | 110 | 6.0 |
| pH | 6.5-10.5 | 8.0 | 9.2 |
| TABLE IV: Analytical Results of Contaminants in Filter Cake | |||
| Metal | TCLP Limits (mg/kg) |
Concentration
in Filter Cake (mg/kg) |
Leachate (mg/kg) |
| Chromium | 5.0 | 4190 | 0.034 |
| Copper | - | 91.0 | 0.036 |
| Iron | - | 88,454 | 0.132 |
| Nickel | - | 759 | 4.0 |
| Zinc | - | 139,000 | 678 |
REFERENCES
1. "Langes Handbook of Chemistry," 11th ed., McGraw Hill, New York, NY,
1973.
2. Amer, S.I. in "Treating Metal Finishing Wastewater," Environmental
Technology, March/April Issue, 1998.
3. "Techniques for Removing Metals from Process Wastewater," Chemical
Engineering, April 15, 1984.
4. Kanluen, R. and Amer, S. I., in "Water Treatment Made Simple," Proceedings
of the 12th IWA-ASPC Regional Conference on "Water Beyond 2000," Chiangmai,
Thailand, Nov. 5-9, 2000.
5. "Toxicity Characteristic Leaching Procedure," (1986) Test Method 1311
in Test Methods for Evaluation of Solid Waste, Physical/Chemical Methods,
EPA Publication SW-846, 3rd ed., (November), as amended by Updates I,
II, IIA, U.S. Government Printing Office, Washington, DC.
Serving the Finishing
Industries. Since 1936.
PFONLINE and all contents are properties
of Gardner Publications, Inc.
All Rights Reserved.