Quantifying Paint System Performance
By George Shaw
Materials Engineering Team
U.S. Army - Tank Automotive and Armaments Command
How do you know the
paint you just bought will actually meet the manufacturer's claims? Because
there is no industry standard for ranking or evaluating paint systems,
you don't.
The U.S. Army Tank Automotive and Armaments Command is in the process
of leveraging commercial technology to improve military coating systems.
Innovative approaches with respect to system design and evaluation are
necessary to provide the military with a vehicle that lasts longer and
requires less maintenance.
We have found that the automotive industry has done an exceptional job developing highly durable coating systems and the test methods to qualify them. In the automotive industry, corrosion and chip resistance are often taken for granted because of the use of galvanized steel, plastic, aluminum and anti-chip coatings. While automotive manufacturers have recognized the value of multi-coating paint systems in conjunction with more corrosion resistant substrates, for the most part, general industry and the military have not made this quantum change. For these industries, total system performance is driven by the pretreatment, or lack thereof, primer and topcoat.
Although some companies have accepted accelerated corrosion testing as a means to establish the durability of their paint/pretreatment system, the durability of an epoxy-based system, based upon accelerated corrosion testing alone, can be extremely optimistic if other safeguards are not exercised.
In the last few years,
this has become a more serious issue with the drive to reduce VOCs. Lower
VOC primers have higher viscosities and are less efficient as corrosion
inhibitors. Consequently, they tend to require greater film thickness
to get the same performance level as their higher VOC versions. Although
a thicker epoxy provides improved corrosion resistance, it is more brittle.
Considering the industry's move toward lower VOC coatings, a thorough
evaluation of multi-coating systems, substrates and pretreatments is necessary.
Therefore, we took the automotive industry's proven methods and made some
slight "improvements" to create a quantitative method for evaluating paint
performance. Many of our new system contracts have paint and substrate
corrosion performance defined in terms of GM 9540P cycles coupled with
a gravelometer test for chip resistance and impact test for process control.
Cleaning & Pretreatment - The Variability Begins
Cleaning and pretreatment are the foundation for the coating system. Therefore,
some general insight into cleaning and pretreatment and the pretreatment
methods we used in this study need to be discussed.
Although a cleaning or pretreatment process might perform well in laboratory conditions on flat test panels, the ability to properly clean and pretreat complex structures with multiple metals and surface conditions, corners, edges, cavities, welds and flash/heavy rust is another matter. Temperature control, concentration and contamination levels in the various recycled chemicals need constant monitoring to maintain minimum levels of performance.
Manufacturing processes, such as laser cutting, forming lubricants and corrosion inhibitors to retard flash rusting are sources of constant grief to the finisher. Laser cut edges resist most pretreatments, and some drawing compounds and corrosion inhibitive oils only come off with specific cleaners. Their removal becomes even more difficult if they have been on the surface for considerable periods of time. Welding processes, which deposit a glassy flux on the weld deposit, provide an unreactive surface for both the pretreatment and the primer.
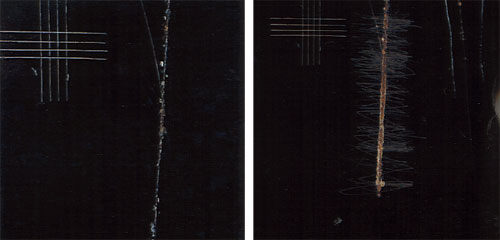 |
|
On the left is a test coupon after an "automotive" evaluation. The image on the right is the same test coupon after it had been scraped. The scribe creep goes from an intermittent condition to an almost continuous response along the scribe line. |
Problems are often compounded when the finisher, due to a lack of manufacturing floor space, chooses to store parts outside with little or no corrosion inhibitors or water-soluble inhibitors. The general and galvanic corrosion cells created by this practice often result in tenacious corrosion products that require both mechanical and chemical intervention for complete removal.
The typical approach
is to use an alkaline cleaner in conjunction with an abrasive blast, iron
phosphate, zinc phosphate, chromate conversion, vinyl wash primer or organo-ceramic
derivative pretreatment. But, chemical cleaning becomes increasingly more
complicated when inorganic contaminants and mixed metals are involved.
In addition, some cleaners have wetting agents, which augment the removal
of certain contaminants but may hide organic contaminant detection by
giving the visual impression of a clean water break. Fish eyes can be
prevented with this practice, but overall long-term performance is compromised.
There is no "easy" way to determine that the surface is free of all inorganic
contaminants, since water break criteria do not apply. Adhesion failures
in an accelerated corrosion test of adequate duration are often the only
reliable means of contaminant detection.
Abrasive blasting is highly regarded by some as providing the optimal
"pretreatment" for large platforms. There is no question that for quickly
removing old coatings, corrosion and mill scale, various media and blast
pressures can be employed to accomplish the task with minimal degradation
to the substrate. Complications, however, do arise when the spent media,
old coatings and metallic fines need to be contained or removed from the
remainder of the structure to preclude primer contamination.
Unless the process is contained in an environmental booth, this process cannot be easily carried out in a manufacturing environment. Although water delivery systems help to contain the residue, a relatively expensive reverse osmosis water system is necessary to preclude the development of almost instantaneous flash rusting on ferrous surfaces using water sources with high mineral salt content.
However, the greatest deterrent to abrasive blasting is its inherently limited ability to develop chemical bonding between the substrate and primer compared to chemical pretreatments. Good mechanical bonding between the primer and substrate can be obtained if the primer is applied shortly after blasting to the proper surface profile.
Product data sheets, which identify a particular level of coating performance in conjunction with abrasive blasting, can be very misleading. Both surface profile and dwell time from blasting to primer application are critical variables. The difference in performance between a 2-hr dwell time (laboratory test coupon) and a 2-day dwell time (typical production) can be dramatic.
Iron phosphating does not meet Army acceptance standards as a pretreatment but is generally an effective cleaner. Numerous attempts have been made in the past to get various manufacturers' products to pass the military's 336-hr salt spray test on scribed panels with an epoxy primer. Not one has succeeded.
The polyvinyl butyral wash primers are recipe driven pretreatments dictated by DOD-P-15328. They contain hexavalent chromium and typically have a VOC of 6 lb/gal with 10% solids. These factors limit its use in some states. These are two-component products containing a phosphoric acid component. They are touted as adhesion promoters. They have no other performance benefits expressed or implied. They have been used on a variety of substrates but require that the film remain wet a minimum of 2 min to allow the acid component to have maximum effectiveness. Furthermore, the wash primer must cure a minimum of 1 hr before primer application (MIL-C-8507). There have been reports that the chromium component leaches through the paint film and has been detected in the drip line of vehicles. This product has been used by numerous manufacturers and is allowable on many Army systems.
Aside from its significant
environmental problems, wash primers suffer from two major weaknesses associated
with their application:
Zinc phosphating and organo-ceramic derivative pretreatments are the preferred method for obtaining maximum corrosion resistance of the coating system on ferrous and galvanized surfaces. Immersion systems provide coverage to internal as well as external surfaces and are the preferred method for large or complex components.
There have been significant advances in the zinc phosphate industry in the last 8 years. Application processes can be run at lower temperature (110F) with less sludging (disposal), which reduces the overall operating cost. Crystal size, morphology and coverage have been optimized to give consistent performance in conjunction with high performance coatings. Functional capabilities have been enhanced.
Low alloy steels previously required a preliminary abrasive blasting operation to remove excessive surface carbon and oxidation products. Failure to perform this operation resulted in surfaces with 20-50% coverage of zinc phosphate crystals and unsatisfactory coating performance. Alkaline cleaning/zinc phosphating technology that can provide the same alloy with 100% crystal coverage at less than 0.5 micron size without the need for abrasive blasting is commercially available.
Improving the chemical robustness of the system has its drawbacks however. Previously both organic and inorganic contaminants often could be detected by the lack of crystal coverage over the contaminated surface on the scanning electron microscope. Now the contaminated surfaces appear to have the same quality and coverage of crystals as the clean surface. It is the bond strength of the zinc phosphate crystal to the substrate that has been compromised. Failure analysis now requires more stealthy analytical methods to determine the type and degree of contamination.
Chromate conversions (MIL-C-5541) have been the benchmark for pretreating aluminum alloys for the military. Due to the hexavalent chromium content in the chemistry, alternatives are being sought.
Corrosion Resistance Testing
To establish benchmarks that could have high statistical correlation to
actual exposure conditions, GM 9540P- Accelerated Corrosion Test (ACT)
was selected as the protocol for establishing corrosion resistance.1
The merit of this protocol is derived from its thermal and moisture cycling,
which induces mechanical stresses in the coating system. In other words,
this test mimics the real-time fatigue of a coating system in a corrosive
environment; it is not just a corrosion test. ACT has become an accepted
industry standard and is a readily available protocol at independent test
laboratories. Test panel substrates were cold rolled steel (CRS), galvanized
steel (G 90), AA 5083-H231 and AA 6061-T6.
Both electrocoat epoxies as well as spray epoxies were evaluated. Products were military approved or commercially advertised as having exceptional levels of corrosion resistance. Seven products from five manufacturers are provided in the listing. It should be noted that comparative data from two additional products was purposely omitted. These two products displayed a significant variation in ACT results at two different test facilities. It is not without coincidence that these same two products have demonstrated significant variations in 336 hr salt spray process control test results over time. The source of the variability cannot be established at this time. It is believed that the variation is more likely attributed to variations in the wet paint (lack of process control, solvent manipulation or additive change or omission by the paint manufacturer) than variations in the test laboratory.
All substrates were cleaned (water break free) and pretreated as noted. Surface profiles were established with replica tape. Some received a combination of both abrasive blasting and chemical pretreatments. One product tested recommended a specific surface profile prior to primer application. Some manufacturers are required to abrasive blast the surface prior to chemical processing due to poor storage/handling conditions that create unacceptable levels of corrosion or purchase hot rolled steel with unacceptable levels of mill scale.
Some test coupons were prepared over knowingly inadequate zinc phosphate pretreatments to assess the impact on performance. An unacceptable pretreatment is considered one that has less than 70% crystal coverage by scanning electron microscope evaluation at 500x. High performance coatings have demonstrated that they are capable of providing adequate protection even with marginal pretreatments. Marginal coatings, however, require almost perfect pretreatment conditions to achieve even a minimal level of performance. Unless otherwise noted, the zinc phosphate pretreatment had 100% crystal coverage. All test panels were cured a minimum of 7 days prior to scribing to assure a fully cured condition.
Testing was conducted at four facilities. In many cases duplicate panels of a particular system were tested at two facilities or at the same facility at different times. Test results were averaged on a minimum of six replicates. Coating dry film thickness was reported as the mean value of the range (six readings per test coupon). The range of coating dry film thickness (dft) was a function of the application process. Electrocoated test coupons had the same dft (relative to dft readings recorded to the nearest 0.1mil) over the entire test surface. On sprayed test panels the range varied from 0.2-1.7 mils on a single panel. Typically, the higher the solids content of the coating the greater the applied range.
A significant limitation of the GM 9540P protocol is the method of evaluation after exposure. The test panels are typically rinsed and subjected to high-pressure air to remove any loose primer; occasionally the air nozzle tip may be used to loosen the corrosion scab. Many coating systems fail by cathodic disbondment. This current automotive method was not considered adequate to address this failure mechanism. Consequently, the evaluation protocol was modified.
All test coupons were scraped at a 30-degree contact angle to the test surface with a blunt-edged, 1.5-inch-wide metal putty knife prior to evaluation. To demonstrate the difference in evaluation techniques, Figure 1 shows a test coupon after an "automotive" evaluation. The opposing image is the same test coupon after being scraped. The scribe creep goes from an intermittent condition to an almost continuous response along the scribe line. The maximum scribe creep measured from one side of the scribe increased from 1.6 mm to 1.7 mm.
The failure criteria for steel and galvanized surfaces was the same criteria used since 1985 by the Tank Automotive and Armaments Command (TACOM) for the system of pretreatment and primer contained in TT-C-490, "Cleaning Methods for Ferrous Surfaces and Pretreatments for Organic Coatings." If any of the following four conditions was met, the test coupon was considered to have reached "failure:"
These criteria are considerably more liberal than most current automotive
standards.
The benchmark for aluminum systems was established from a prior internal study using two aluminum alloys, two epoxy primers and two thickness criteria. 160 cycles of GM 9540P were required on AA 5083 and AA 6061 test coupons with a chromate conversion before any primer could be scraped from the scribe. There was no degradation in the field.
The test coupons were generally kept in the ACT cabinet until failure criteria had been met (test to failure). When the corrosion scab or paint eruption met the 0.125 inch criteria, the coupon was scraped. Some coupons were removed at a predetermined stop point such as 40 or 80 cycles.
The epoxy coating on the galvanized and aluminum coupons normally does not erupt around the scribe line as on CRS. These materials must be scraped to determine coating failure.
|
Substrate |
MIL-C-5541 |
Abrasive Blast (MIL) |
Wash Primer
X X X |
Zinc Phosphate X |
Primer ID |
VOC (lb/gal) |
dft |
Cycles to Failure |
Comments
0.0 scribe creep 0.0 scribe creep 0.0 scribe creep 0.03-in scribe creep 0.0 scribe creep 96-hr delay after blast before prime 0.07-in scribe creep 0.03-in scribe creep field failure field failure poor quality treatment |
Notes:
|
|||||||||
Once the coupon was scraped, the test panel was retired from further corrosion testing. If the failure criteria had not been met at this interval, a plus (+) designation was added to the duration. Noting the scraping procedure, the amount of scribe creep could be considerably greater than that visually observed in the cabinet at the point of removal. Those coupons whose scribe creep increased by scraping more than 0.031(1/32) inch than that recorded prior to scraping were so noted by an asterisk following the number of cycles. The number noted represents a deduction of 4 cycles for every 0.031 inch increment greater than 0.156 inch of scribe creep (for example, a maximum scribe creep of 0.236 inch after scraping would have 10 cycles deducted from the original rating).
Beyond Corrosion Resistance
An appropriate engineering decision could be made with respect to a given
coating system from this data alone only if the following two conditions
are met: 1) the coated product was in a static application not subject
to flex or chipping conditions and 2) the product was not subject to direct
sunlight (UV exposure).
|
Primer ID* |
dft |
Direct Impact (in-lb) |
Reverse Impact (in-lb)**
160 160 160 6 < 4 4 < 4 6 < 4 8 < 4 < 4 < 4 |
| * VOC of H3,
N2 and S2 primers is 3.5 lb/gal ** A coating with an impact value less than 6 in-lb is categorized as brittle (ASTM D 2794). All test coupons were a minmum of 30 days. |
|||
Although epoxies are known for their exceptional corrosion and fluid resistance they suffer from two weaknesses. Epoxies show an inverse relationship between dft and flexibility. Consequently, if you need to increase the dft to get corrosion resistance, you are compromising chip resistance in the process. Various tests are used by the industry to assess flexibility of the coating. Those that best accommodate high strain rates reflective of stone pecking damage are the impact tests per ASTM D2764, STM for Resistance of Organic Coatings to the Effects of Rapid Deformation (Impact) and the various automotive gravelometer tests based upon SAE J400, Test for Chip Resistance of Surface Coatings. A comparison of five epoxies with respect to direct and reverse impact is provided in Table II. The criteria for rating were no cracking by unaided visual observation or disbond (tape) of the coating.
A more insidious problem with epoxies is their poor UV resistance, which degrades the performance of the coating (commonly referred to as chalking) via direct degradation or photolytic oxidation.2,3 Although it is general knowledge that you don't expose epoxies to UV, there is the belief that applying a topcoat will always shield the epoxy. This assumption can have devastating consequences. There is no accepted method for accelerating UV exposure with a real-time correlation. The best protocols are developed around xenon arc or the use of mirrors to track and intensify light exposure in a desert climate coupled with moisture. Many use the UV test protocols to measure changes in color or gloss over time. The serious damage occurs when the topcoat is transparent to UV transmission allowing the epoxy to degrade and generate a disbond between the primer and topcoat.4
Many manufacturers have consequently added proprietary UV absorbers or reflective pigments in their topcoats to protect the epoxy. As the user can't readily verify this addition there must be considerable trust in both the topcoat suppliers chemistry and manufacturing controls to provide a consistent, compliant product. Adding excessive pigment to a topcoat formulation (flat gloss) in the belief that it will block the UV photon is not a valid remedy.
Two problems are associated
with flat topcoats:
Results
Based on this research, we can draw some definite conclusions:
The reliance on the
salt spray test (which has no correlation to real time exposure) for product
development and qualification may be responsible for many misconceptions
in the non-automotive coatings industry. Coating formulation is highly
complex chemistry, and the degradation mechanisms are no less complex.
The formulator at a minimum must have knowledge and control of the molecular
weight of the resin, crosslink density of the cured polymer, plasticizer
and surfactant additives and pigment type/concentration. These factors
have a major impact on the adhesion, corrosion resistance, fluid resistance
(for example, water, gasoline, hydraulic fluid or decontamination solvent)
and flexibility of the final film. The coating chemist must rely on meaningful
physical testing to confirm the actual performance of his engineered product.
The variables involved and possible synergisms, either positive or negative,
are too complex to intuitively predict any outcome.
The writer would like to acknowledge the following individuals for
their assistance in developing the test data for this project: Shawn Dolan,
Henkel Surface Technologies; John Shaffer, Cathy Trusik and Varina Andriaschko,
PPG Industries; and Tom Braswell, UDLP.
| REFERENCES: 1. L.A. Roudabush, H.E. Townsend and D.C. McCune, Update on the Development of an Improved Cosmetic Corrosion Test by the Automotive and Steel Industry, SAE 6th Automotive Corrosion and Prevention Conference, Dearborn, MI, Oct 4-6 1993 2. M. P. Diebold, Unconventional Effects of TiO2 on Paint Durability, E.I. Du Pont de Nemours, Wilmington, USA 3. C. H. Hare, Mechanisms of Photolytically Induced Degradation, Journal of Paints, Coatings and Linings, March 2000 4. D.R. Bauer, Chemical Criterion for Durable Automotive Topcoats, Journal of Coatings Technology, August 1994 5. V. McGinniss, M.S. Donley and B. Metz, Degradation Mechanisms and Performance Metrics for Aircraft Topcoatings, DOD/Industry Aerospace Coatings Conference, Monterey, CA, May 1999 6. M.R. Van De Mark, Coatings R&D Notebook, Industrial Paint and Powder, Feb 2000 7. D.J. Weinmann, B.T. Chang, Performance of Flexible Epoxy Coatings for Offshore/Marine Applications, Shell Resins and Versatics, Houston, TX |
Serving the Finishing Industries. Since 1936.
PF Online and all contents
are properties of Gardner Publications, Inc.
All Rights Reserved.